Alkali metals are good reducing agents as they readily lose their electrons due to low ionization enthalpy. A Ni B Mg C Fe D Co 2.

Solved Part A Which Of The Following Metals Is The Best Chegg Com
So while all equations above show zinc as the reducing agent the equations with Mg show Mg as the reducing agent.

. Lithium which has a high ionisation energy value in alkali metals serves as the solutions main reducing agent. Reducing agent - Element which loses their electrons to an electron recipient in a redox reaction are known as reducing agent. Which of the following metals is the best reducing agent.
Which of the following metals is the best reducing agent. The metal ion with lower reduction potential acts as the best reducing agent with corresponding metal The reduction potentials are as follows. A Co 2 B Zn 2 C Cd 2 D Cu Thank you for the help.
Therefore Al acts as a best reducing. Skip to main content. See the answer See the answer done loading.
Both of the half-cell reaction for Au are higher than the half-cell reaction of all the other element so I think use either one to compare is okay. Answer 1 of 9. Which of the following metals is the best reducing agent.
A Ni B Mg C Fe D Co 2. LiAlH4 is one of the strongest reducing agents known. Which of the following metal cations is the best oxidizing agent.
Answer to Solved Which of the following metals is the best reducing. Which of the following metals is the best reducing agent. Among all the alkali metals Lithium L i is the strongest reducing agent in aqueous solution.
You just need to arrange it so that the most negative one is the strongest reducing agent. Who are the experts. Which of the following metals is the best reducing agent.
E Melting and boiling points of metals and non-metals. Who are the experts. Rank the four metals Cu Zn Mg and Pb from the strongest reducing agent to the weakest reducing agent.
Fe Cd Li Co. The best reducing agent is lithium with the maximum negative value of electrode potential. D Elements with a more metallic character form acidic oxides and with more non-metallic character form basic oxides.
Which of the following metals is the best reducing agent. Want to see the step-by-step answer. Using your data from Table 2.
You are told that metal X is a better reducing agent than metal Y. This must mean that. There are a few metals that do not just dissolve in any acid but need an acid such as HNO3 whose anion is a strong oxidising agent instead.
Recall that by strong reducing agent we mean it causes another metal to be reduced. Li is the most strongest reducing agent and two factors are responsible for this 1ionisation energy Ionisation energy should be low for a good reducing agent but due to small size of Li it is high so according to this Li should not. The best reducing agent is Na and the weakest is Cl-.
C Alkalis and alkaline earth metals react with water liberating oxygen. Which of the following is the best reducing agent. The reducing agent after losing electrons gets oxidized and also causes the other reactant to get reduced via providing electrons.
Consider the galvanic cell shown below the contents of each half-cell are written beneath each compartment. Reducing agents have a tendency to give away electrons. Secondarily and qualitatively you can remember that alkaline earth and alkaline metals both give up their electrons easily have low electron affinity and can be considered to have good.
Thus Mg is a stronger reducing agent. Metal hydrides such as NaH CaH 2 and LiAlH 4 which formally contain the H - ion are also good reducing agents. Popular reduction agents include potassium calcium barium sodium and magnesium metals as well as H-ion.
This problem has been solved. 2Au s 3Zn s 4Cu s 3Zn. By convention reduction potential or the propensity to be diminished are the normal electrode potentials.
Most metals are thus strong reducing agents. The metals of the s-block in the periodic table are stated to be top reducing agents. Characteristics of reducing Agent.
Which of the following metals is the best reducing agent. A strong acid contains free protons using the 201 table compare the metals to the reduction of protons to H2 gas using the table of reduction potentials to see which is more favored to be oxidized when compared to hydrogen. A Mn b Al c Ni d Cr.
Good reducing agents include the active metals such as sodium magnesium aluminum and zinc which have relatively small ionization energies and low electro-negativities. See the answer See the. Check out a sample QA here.
Chemistry questions and answers. Most positive E--most negative E. Some compounds can act as either oxidizing agents or.
Experts are tested by Chegg as specialists in their subject area. O Mn O Mg Cr Cd check_circle Expert Answer. Cr 3e--Crs E-073 V Mn2 2e-Mns E-118 V Cd2 2e Cds E-040 V Here Al3 contains the lowest reduction potential than other provided metals.
Answer 1 of 8. Mn O Mg O Cr O Cd. Which of the following metals is the best reducing agent.
Which of the following metals is the best reducing agent. A Co2 B Zn2 C Cd2 D Cu Thank you for the help. A reducing agent is an element which loses an electron to another chemic.
LiHNaBH4 are also strong reducing agents. Chemistry questions and answers. We review their content and use your feedback to keep the quality high.
As opposed to itself. Which of the following metal cations is the best oxidizing agent. Which of the following metals is the best reducing agent and which is the worst.
Weak reducing agen--strong reducing agent.
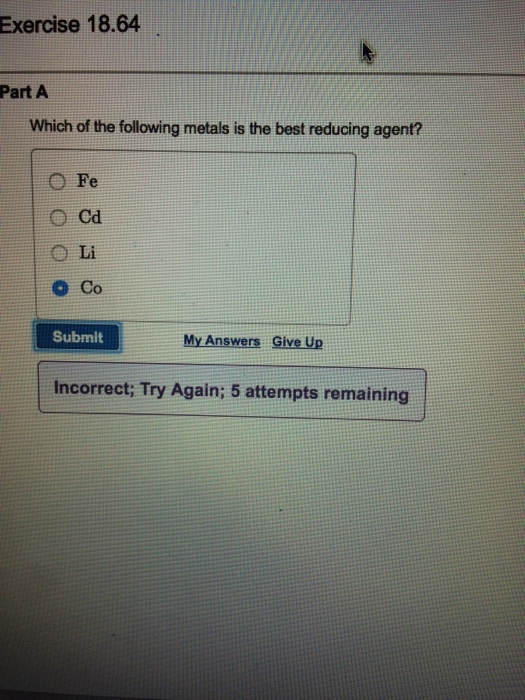
Solved Which Of The Following Metals Is The Best Reducing Chegg Com
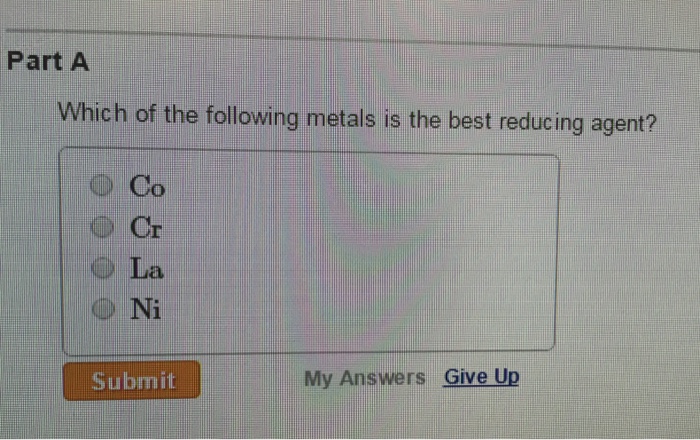
Solved Part A Which Of The Following Metals Is The Best Chegg Com
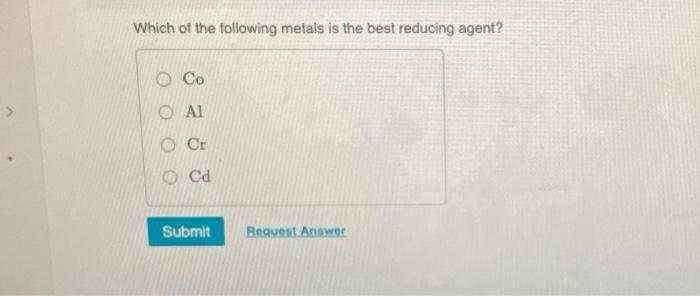
Solved Which Of The Following Metals Is The Best Reducing Chegg Com

Solved Which Of The Following Metals Is The Best Reducing Chegg Com
0 Comments